How modelling can maximise the efficacy of cancer immunotherapy
Recently, combination treatments involving immunotherapy have emerged as an exciting new way to tackle cancer [1].The spotlight has fallen especially on immune checkpoint inhibitors. In this post, we discuss how modelling can be used to unlock key insights to help optimise the promise of combination therapies.
Immunotherapy as a treatment approach has revolutionised oncology – and is one of our key areas of focus here at Physiomics. Huge breakthroughs have been made in this complex field in the last few decades, from administering the first combination immunotherapy (nivolumab plus ipilimumab) in melanoma patients, to the recent approval of brexucabtagene autoleucel, a CAR T-cell immunotherapy for lymphoma.
Traditionally cancer has been treated by attacking it with chemotherapy and radiation, or by removing it with surgery. Immunotherapy – which enhances the immune system’s ability to target and fight cancer cells – presents a new, universal way to tackle cancer. With the number of new cases on the rise globally [2], it is now established as an important pillar of cancer treatment.
Unlocking the potential
The efficacy of many cancer immunotherapies such as T-cell therapy, cytokines, cancer vaccines, oncolytic viruses and monoclonal antibodies, has been demonstrated in recent years [3]. Immune-checkpoint inhibitors – which stimulate T cells to fight cancer – have generated tremendous interest in particular and are helping to provide new, smarter treatments, including for prostate cancer [4].
But not everyone responds to these therapies; in most patients, the cancer either doesn’t respond fully to immunotherapy drugs or becomes resistant [5]. The aetiologies of this resistance derive from an array of factors such as the nature of the tumour itself alongside its complex interplay with a potentially immunosuppressive microenvironment. Excitingly, immunotherapy combinations are helping to unlock this potential.
Enhancing treatment: immunotherapy combinations
The combination of immunotherapy with Radiation Therapy (RT), for instance, is an actively growing field of clinical investigation [6]. Research has shown that combining radiotherapy with immunotherapy can overcome immune suppression to make treatment more effective [7].
The combination of immunotherapy drugs with other immunotherapies presents another interesting modality in this regard – with the ability to further enhance anti-tumour efficacy [8]. A clinical trial using a combination of two checkpoint inhibitor drugs, ipilimumab and nivolumab, recently showed remarkable results in treating metastatic melanoma, a disease long regarded as untreatable [9].
This unique potential for immunotherapy combinations can be best demonstrated through what is rightly regarded as one of the most incredible breakthroughs of recent years. James P Allison and Tasuku Honjo’s Nobel prize winning work on cancer immunotherapy were the first to identify the proteins PD-1 and CTLA-4 as targets for cancer treatment. And today many companies are developing drugs that can block CTLA-4, PD-1 and PD-L1. Ipilimumab was the first of its kind to emerge from Allison’s discovery.
Immune-checkpoint therapy against PD-1 has proven particularly effective for several types of cancer. More interestingly, clinical studies indicate that combination therapy, targeting both CTLA-4 and PD-1, can be even more effective in releasing the brakes on the immune system and triggering its inherent ability to destroy cancer cells [10].
These breakthroughs are a testament to the incredible potential for immunotherapy combinations to fundamentally change the outcomes for patients with advanced cancer.
But with such great power comes great complexity. While combination immunotherapy drugs offer new hope, successfully designing combination treatments relies on identifying the best balance between risk and benefit [11].
Optimal dosing: getting it right
Historically, cancer treatment has been aimed at destroying as many cancer cells as possible through chemotherapy or radiation. But with new drugs such as immunotherapy, there is now a greater focus on creating treatments that are more precise and targeted.
Immunotherapy has shown it can help conventional treatments work better. But the tumour microenvironment is a highly dynamic network. To fully maximise the benefits of these combination treatments, identifying the appropriate dosage, timing and sequence of administration are all critical.
This is particularly important because different therapies interact with the immune system in different ways. More importantly, they act in a variety of dose- and time-dependent ways to potentiate immunotherapy.
Radiotherapy in particular – historically considered to be immunosuppressive, and a local form of cancer treatment – has a complex and conflicted relationship with the immune system and multiple interactions with the tumour microenvironment.
Today, mounting evidence demonstrates that RT can induce strong anti-tumour responses [12] – and perhaps most interestingly, it can create these responses outside of the targeted site [13]. This is called the abscopal effect.
Triggering the abscopal effect
The abscopal effect is a fascinating phenomenon with huge implications for the management of cancer. It occurs when local therapy such as radiation not only shrinks the targeted tumour but also leads to the shrinkage of untreated tumours elsewhere in the body. Abscopal responses have been documented in various types of cancer, including melanoma, breast and lung cancer [14].
There is now growing evidence that adding immune-checkpoint inhibitors to radiation boosts the chances of an abscopal response in patients, compared with the use of radiation alone. What remain unclear are the best dose and dose fractionation to maximize immune activation and trigger the therapeutic benefits of this extraordinary effect [15].
This is where mathematical modelling can add tremendous value. Mathematical models informed with experimental data can simulate such experimentally untested protocols to help identify the optimal radiotherapy protocols to trigger the immune response [16].
Identifying these protocols has huge significance given checkpoint inhibitors are now the most frequently prescribed immunotherapy [17]. Meanwhile, about 50% of all cancer patients receive radiotherapy as part of their treatment [18].
Similarly, sequence of administration has been shown to also influence the success of combination treatments. Treatment with immunotherapy drug ipilimumab after delivery of radiotherapy, for instance, was shown to generate a partial response in only 18% of patients with metastatic melanoma in a phase I study [19]. However, administration of ipilimumab before radiotherapy remarkably generated a significantly greater irradiation response in these patients [20].
Studies such as these point to the importance of identifying the optimal dosing schedule and sequence of administration to unlock the full potential of immunotherapy combinations.
Immunotherapy combinations in themselves can potentially reduce drug resistance, while providing therapeutic anti-cancer benefits, such as reducing tumour growth and metastatic potential. Further, the five-year survival rates for most metastatic cancers are still quite low, and combination therapies have proven they can increase prognosis for these patients [21].
How Physiomics can help
In the past few decades, knowledge about the relationship between cancer and the immune system has grown quickly. Meanwhile, vast technological advances mean we now have the capabilities to deliver key insights to simulate the effects of immunotherapy drugs on cancer cells and predict the effects of drug combinations. Thus, we can optimise dosing schedule in a reliable way and attain truly effective therapies for cancer, which remains one of the world’s most pressing healthcare challenges.
In our last post, we discussed the importance of the work currently being carried out in cancer drug development and the importance of continuing these efforts despite the COVID-19 crisis. This includes immunotherapy, which has extensive potential to change the way cancer is managed. Modelling further enhances these abilities and shows where the true potential lies, and which treatments can be more effective.
Today we know that immunotherapy holds the potential to induce durable responses in patients (there are over 2000 ongoing trials investigating anti-PD-1/anti-PD-L1 drugs alone) [22]. We also know that combinations of immunotherapy have superior potential.
Physiomics’ Virtual Tumour™ (VT) platform is an integrated PK/PD simulation platform that can be used to optimize drug dosing and scheduling, and to design new combination therapies. The technology works by modelling the way that individual cells behave within a tumour population.
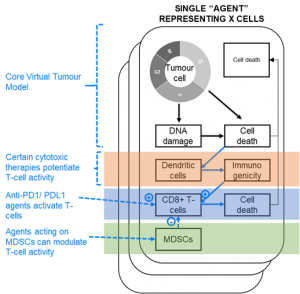
The model has recently been developed to allow simulation of immune-checkpoint blockers with other immunotherapies, to help predict optimal dosing and scheduling of novel immuno-oncology combinations. Modelling the effects of these treatments can enable robust decision-making during early clinical trials.
For more information about our Virtual Tumour™ simulation tool and how it can be used to support oncology R&D, please feel free to contact us and we’d be happy to arrange a brief consultation for you with a specialist in our team.
References
[1] https://www.who.int/cancer/resources/keyfacts/en/
[2] https://www.who.int/cancer/resources/keyfacts/en/
[3] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5481296/#!po=1.42857
[4] https://www.cancerresearch.org/immunotherapy/cancer-types/prostate-cancer
[5] https://www.bmj.com/content/365/bmj.l1824
[6] https://jitc.bmj.com/content/4/1/51
[8] Galon, J. & Bruni, D. Nature Reviews Drug Discovery (2019).
[10] https://www.nobelprize.org/prizes/medicine/2018/press-release/
[11] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6039198/
[12] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6275030/
[13] https://jitc.bmj.com/content/4/1/51
[15] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6275030/#!po=6.52174
[16] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6275030/
[17] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6306034/
[18] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6275030/#!po=6.52174



