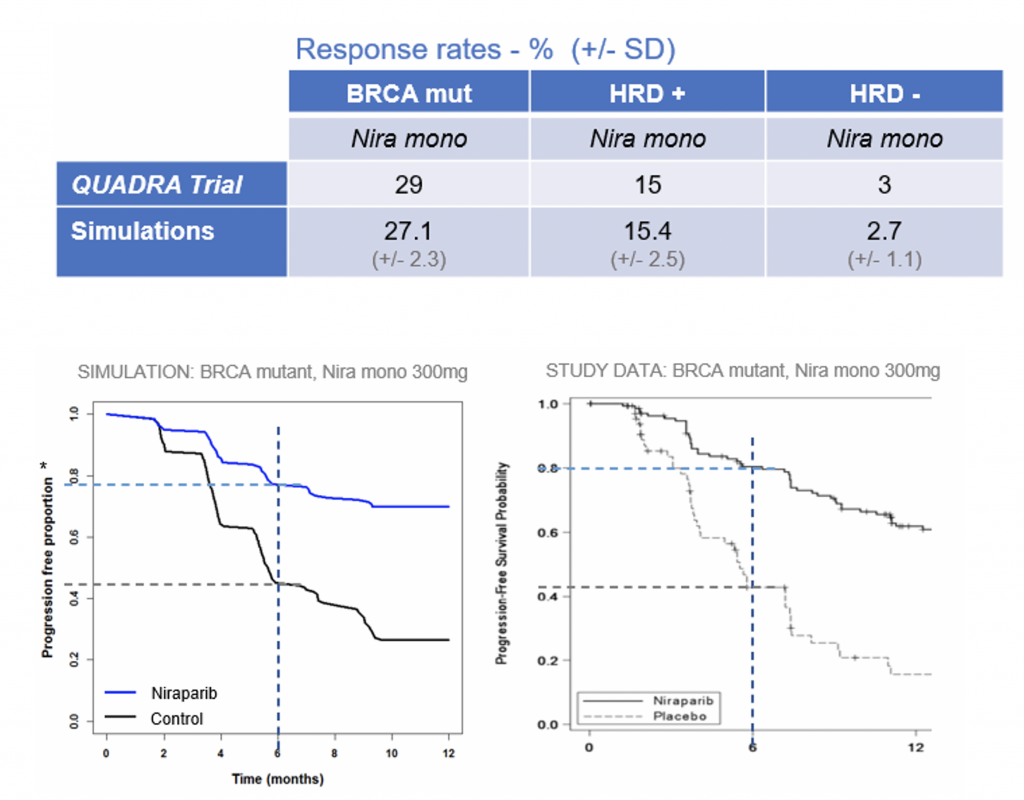
Clinical trial outcomes can be simulated using computational models consistent with your drug’s MOA which incorporate available preclinical and clinical data as well as variability within a clinical population.
This modelling technique is a powerful tool to explore doses, schedules and combinations not yet tested clinically, guide trial design and improve success rate in the clinic.