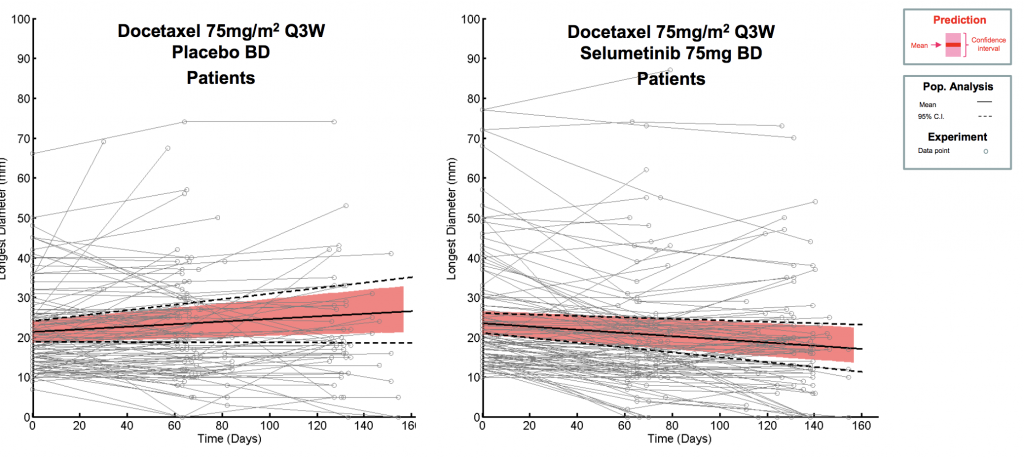
Translational modelling allows you to make the best use of all relevant pre-clinical data to generate accurate human dose predictions and maximise probability of clinical success.
Translating pre-clinical PK and PD data to application in humans is a key milestone in the development of therapeutic drugs.